Psychedelic Alpha’s 2025 Bullseye: Charting the Next Phase of Psychedelic Therapy

Psychedelic Alpha, a UK-based data platform and media known for tracking psychedelic drug clinical research, has released its Q3 2025 Bullseye Charts. It offers the most comprehensive view yet of the global psychedelic drug trials pipeline. Compared to the first Bullseye snapshot by PA in 2022, the field has expanded dramatically, with dozens of compounds now advancing through Phase 2 and Phase 3. The key insight is that psychedelic therapy for depression, anxiety, PTSD, and substance use disorders is no longer confined to a handful of molecules — it has evolved into a diversified, evidence-driven clinical landscape.
How to Read Clinical Trials: Context and Tracker
Psychedelic Alpha has been publishing psychedelic clinical trials updates over the past couple of years. These recurring snapshots are based on The Psychedelics Drug Development Tracker and visualise how compounds progress through different stages of clinical research. They help measure the maturity of the pipeline, track regulatory momentum, and demonstrate the rapid progress of psychedelic therapies toward approval.
Research stages, based on the Tracker:
- Discovery — the earliest stage, when laboratories screen thousands of molecules to identify those with therapeutic potential. Only a handful progress to become “lead candidates.”
- Preclinical — safety and efficacy tested in the lab and in animal models. Compounds that perform well in this setting may be cleared for human trials.
- Phase 1 — first-in-human studies usually include volunteers without diagnosed conditions. The goal is to determine safe dosage ranges and monitor any potential side effects that may occur.
- Phase 2 — the first patient studies, testing safety and preliminary efficacy. Numbers are small, but the results inform how to design larger Phase 3 trials.
- Phase 3 — pivotal trials with large patient groups, often international and multi-centre. At least two successful Phase 3 studies are usually required before regulators consider approval.
- Approval — regulators such as the FDA in the U.S. review all available data and decide whether the treatment can be marketed.
In the Bullseye Charts by PA, inner rings represent compounds closer to approval, while outer rings capture early discovery and preclinical work. This framework is essential for interpreting trends in psychedelic-assisted therapy and understanding where each candidate currently sits in the development pipeline.
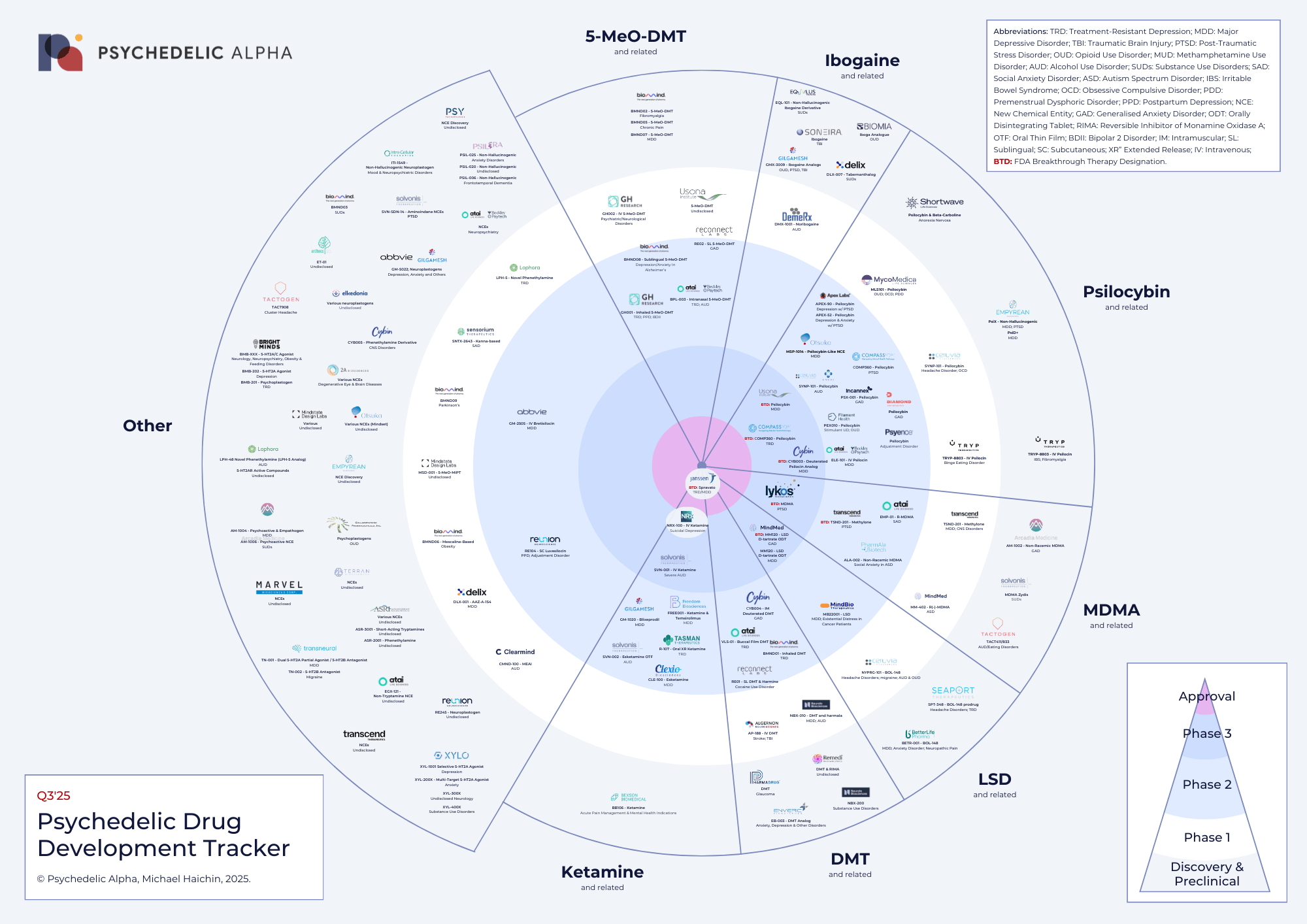
Psychedelic Drug Trials: Classics Rise, New Wave Expands
As of Q3 2025, the global pipeline includes over 200 active psychedelic clinical trials, spanning depression, anxiety, PTSD, addiction, and neurological disorders.
Approval:
- Spravato (nosal spray with esketamine) by Janssen is already the only substance authorised for treatment-resistant depression (TRD) and suicidal depression.
Phase 3 Trials:
- COMP360 (psilocybin) by Compass Pathways — pivotal studies in TRD and PTSD, among the most extensive late-stage psychedelic programs to date.
- MDMA by Lykos (formerly MAPS PBC) — final-stage trials in PTSD with intense Phase 3 results expected to drive the first potential FDA approval of MDMA-assisted therapy.
- MM-120 (LSD) by MindMed — Phase 3 for generalised anxiety disorder (GAD) and additional programs in major depressive disorder (MDD).
- NRX-100 by NRx Pharmaceuticals and SVN-001 by Solvonis are exploring intravenous ketamine infusions for depression and severe alcohol use disorder.
- Psilocybin programs led by Usona and Cybin are also progressing toward Phase 3 endpoints in major depressive disorder (MDD).
Phase 2 Trials Highlights:
- 5-MeO-DMT compounds (GH Research, Beckley Psytech, Reunion) — tested in TRD, postpartum depression, alcohol use disorder, and Alzheimer’s-related anxiety.
- Psilocin analogues such as Cybin’s CYB003 and Beckley’s ELE-101 — designed for MDD and a faster onset of action than traditional psilocybin.
- Ibogaine derivatives (DMX-1001, EQL-101) — under investigation for opioid use disorder and traumatic brain injury.
- Novel empathogens (methylone TSND-201, ALA-002) — targeting PTSD and social anxiety in autism spectrum disorder.
- Next-generation ketamine formulations (oral XR R-107, IV NRX-100) — for TRD and suicidal depression.
Early-stage Phase I and Preclinical trials also include dozens of new chemical entities (called NCEs), particularly non-hallucinogenic neuroplastogens — brain-rewiring treatments — intended to deliver the benefits of psychedelic-assisted therapy without perceptual effects.
Several compounds carry the FDA Breakthrough Therapy Designations badge, including psilocybin (COMP360), MDMA, LSD (MM-120), and esketamine. These designations emphasise both the scientific promise and regulatory relevance of the field.
Psilocybin and Next-Gen Molecules Take the Lead
Psilocybin and psychedelic mushrooms — 20 programs ≈ 17.1%
Trials led by Compass Pathways, Usona Institute, and Cybin keep psilocybin at the centre of research into major depressive disorder (MDD), treatment-resistant depression (TRD), and post-traumatic stress disorder (PTSD). Psilocybin remains the most clinically advanced of the classic psychedelics.
DMT and 5-MeO-DMT — 19 programs ≈ 16.3%
GH Research, Beckley Psytech, and innovators like Cybin (CYB004) and atai (VLS-01) are testing short-acting tryptamines across TRD, postpartum depression (PPD), and substance use disorders. These compounds are notable for their ultra-rapid onset and brief duration.
MDMA and empathogens — 9 programs ≈ 7.7%
Lykos Therapeutics is conducting pivotal trials of MDMA-assisted therapy for PTSD, while next-generation empathogens such as ALA-002 and TSND-201 are in Phase 2 for social anxiety, including in autism spectrum disorder.
Ketamine and analogues — 9 programs ≈ 7.7%
Spravato (esketamine) remains the only psychedelic-derived drug approved by the FDA. Still, new formulations are moving forward: Solvonis’ ketamine SVN-001 for alcohol use disorder and NRx’s NRX-100 intravenous ketamine for suicidal depression.
LSD derivatives — 6 programs ≈ 5.1%
MindMed leads with MM-120, a Phase 3 program for generalised anxiety disorder and major depressive disorder, while MindBio explores LSD applications in depression and existential distress among cancer patients.
Ibogaine and related compounds — 6 programs ≈ 5.1%
Developers such as DemeRx and Gilgamesh Pharma are advancing ibogaine derivatives into trials for opioid use disorder (OUD), alcohol use disorder (AUD), PTSD and traumatic brain injury (TBI). This once-marginal compound is now gaining traction as a serious clinical candidate.
Novel non-hallucinogenic neuroplastogens— 48 programs ≈ 41.0%
Companies like Reunion Neuroscience, AbbVie, Lophora, and BioMind Labs are exploring “brain-rewiring treatments” without hallucinatory effects. These next-generation compounds represent a potential path to more scalable therapies.
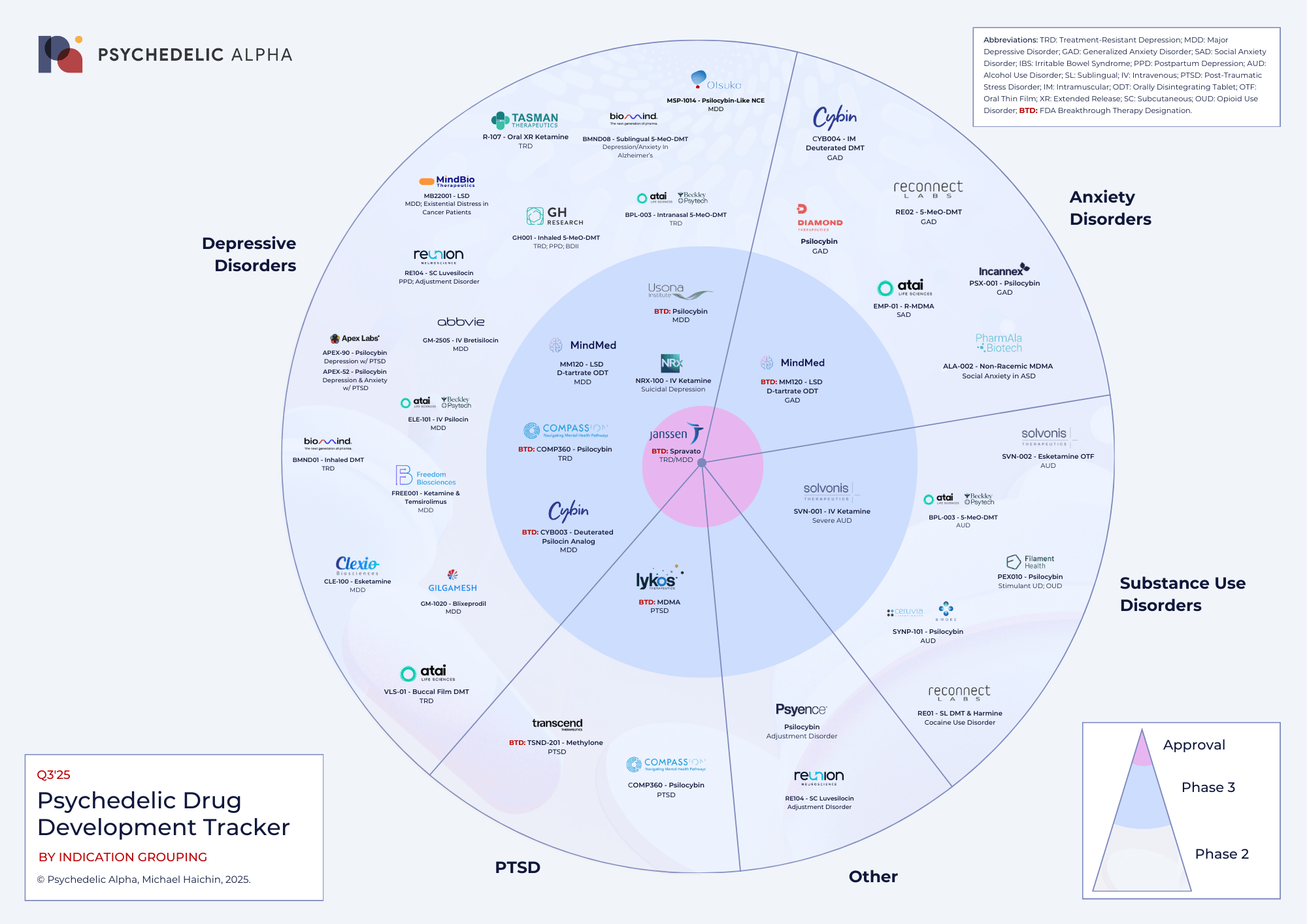
Depression in Focus, Anxiety and Addiction Join the Spotlight
Depressive Disorders ~40% of active programs
Depression remains the central focus of psychedelic clinical trials, with nearly half of all programs targeting major depressive disorder (MDD), treatment-resistant depression (TRD), or suicidal depression. Spravato (esketamine) is already approved for therapeutic use, underscoring that ketamine therapy for depression is the most advanced area. Psilocybin and LSD are among other candidates in Phase 3.
Anxiety Disorders ~20% of programs
Anxiety conditions are the second most common indication, including generalised anxiety disorder (GAD), social anxiety disorder (SAD), and anxiety related to Alzheimer’s disease or autism. Phase 3 programs include LSD trials, while Phase 2 programs range from psilocybin and DMT analogues to empathogens. Evidence for psychedelic therapy for anxiety is growing rapidly.
Substance Use Disorders (SUDs) ~15% of programs
Alcohol use disorder (AUD), opioid use disorder (OUD), cocaine use disorder, and stimulant use disorder are all targets of current psychedelic trials. Active clinical phases include ketamine and psilocybin solutions for severe AUD, as well as DMT + harmine for cocaine use disorder. These trials test whether psychedelics can break cycles of addiction where conventional therapies often fail.
PTSD ~13% of programs
Post-traumatic stress disorder remains one of the flagship indications for psychedelic therapy, especially with empathogens. Lykos Therapeutics is leading pivotal Phase 3 trials of MDMA-assisted therapy for PTSD, while methylone and psilocybin are advancing through Phase 2.
Other Indications ~12% of programs
A smaller set of programs targets conditions beyond psychiatry’s “big four.” These include adjustment disorder — a stress-related condition that happens when someone struggles to cope with significant life changes, like divorce or job loss. While fewer in number, these trials highlight the exploratory breadth of the psychedelic field.
2026 Milestones: Psilocybin and MDMA Phase 3 Results
Major late-stage results are approaching. Phase 3 readouts for COMP360 psilocybin trials and MDMA therapy are expected in the second half of 2026, with the potential to deliver new FDA approvals for psychedelic-assisted treatments.
At the same time, a new wave of non-hallucinogenic psychedelics is emerging. This approach aims to expand access by reducing stigma and logistical barriers, while helping patients integrate insights from their treatment experiences into long-term recovery. It would mark a turning point, moving psychedelic therapy from clinical trials into mainstream medical practice.